Drugs that work – personalised medicine brings a healthcare revolution
on LinkedIn:
It took 13 years and £2 billion to sequence the human genome back in 2003. Fast forward 15 years to today and next-generation sequencing (NGS) can do it for less than $1,000 in a matter of days. High-throughput sequencing technologies, computational power and data-mining techniques have opened up a whole new era in medical treatment – and our approach to product development.
Genetic differences in DNA allow scientists to determine how a patient will respond to certain drugs, enabling doctors to target their treatment. For example, the Sanger Institute recently discovered that the aggressive blood cancer acute myeloid leukaemia could be classified as 11 distinct disease groups, based on specific constellations of genetic mutations. This explains why some patients will be cured and others will not if they receive exactly the same treatment.
This ‘personalised’ approach promises to improve patient outcomes as the right treatment can be given from the start – time is not wasted by finding what works by trial and error – and unpleasant side effects can be minimised. Treatment is more efficient – and less money is wasted on ineffective drugs.
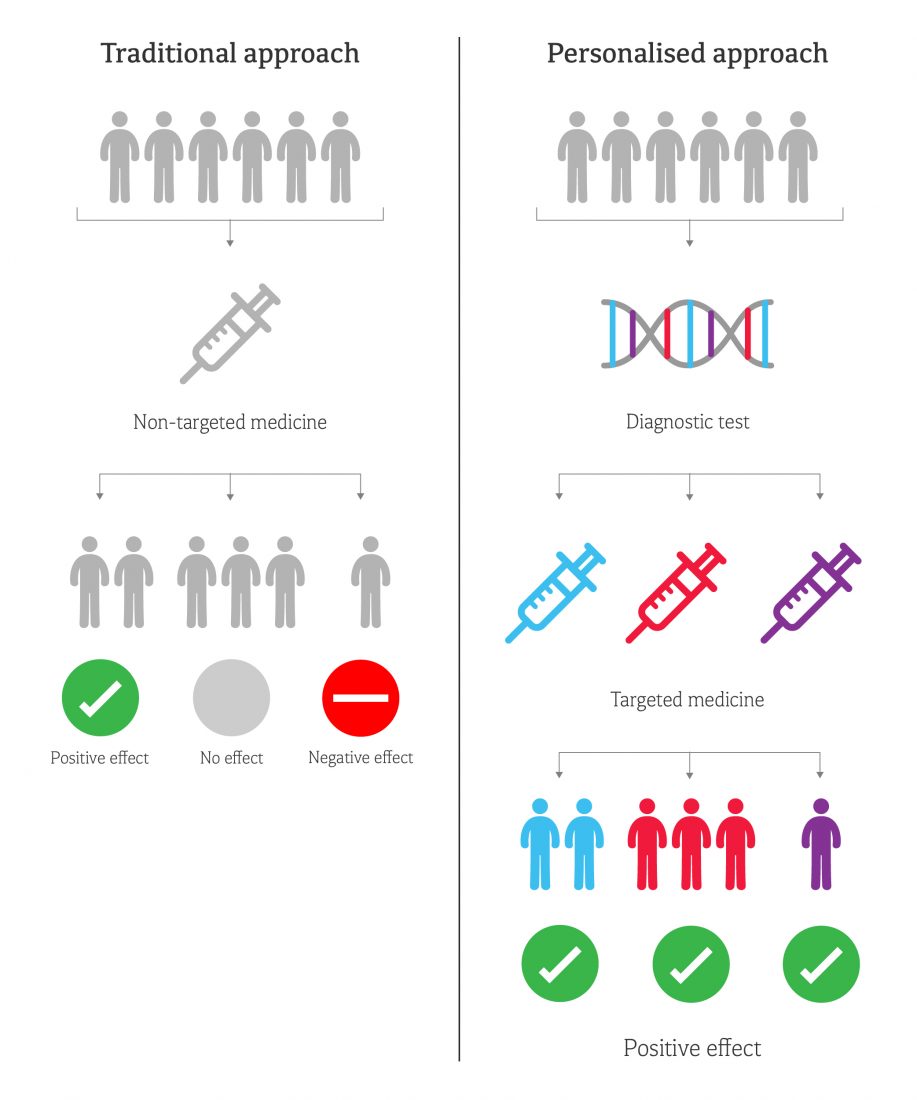
New targeted approaches based on genetic information are gaining particular attention from pharma companies, as they can dramatically reduce drug development costs and timescales. Whereas traditional drug discovery often leads to high failure rates in phase 2 or 3 trials, targeted treatment allows smaller trials and shorter regulatory review times because the drugs are safer and more effective.
Although the market size is smaller, lower side effects mean an increased price can be charged for the drug. An example of this is the Food and Drug Administration’s (FDA’s) approval in 2012 of a new cystic fibrosis (CF) therapy for patients with a rare genetic mutation (G551D mutation). This particular gene is responsible for only 4% of CF cases in the US – around 1,200 people.
The UK government has also recognised the value of the personalised treatment approach. The NHS is undertaking the ‘100,000 Genomes Project’ – 100,000 whole human genomes from 70,000 patients are being sequenced to identify potentially new diagnostics and drive the development of new drugs.
But the race is on. Genomics and biotechnology company 23andMe – which originally provided ancestry information from a saliva sample sent through the post (direct-to-consumer genetic testing) for £125 – has recently been approved by the FDA to provide risk information for 10 genetic diseases, such as Parkinson’s disease and late-onset Alzheimer’s disease. It is estimated that the company has accumulated valuable genetic information about more than two million people.
From this vast library of genomic information becoming available, new products will emerge that target the underlying cause specific to an individual patient. This will likely involve at least two medical products – a diagnostic test and the therapeutic product itself, working together as a so-called companion diagnostic (a diagnostic that is essential for the safe and effective use of a corresponding drug).
Pharma and device companies will need to collaborate more closely to ‘co-develop’ these products to ensure the drug is safe and effective, and the performance of the diagnostic is acceptable. And, since the barriers to drug development are significantly reduced with targeted treatment, smaller innovative companies will get involved. With its access to hugely valuable genomic data, 23andMe is one such company – which is presumably why it has just raised $250 million from private investors.
I predict that NGS and data analytics will be the powerful research tools that provide the understanding. But although NGS is starting to move out of the research lab and into the clinical environment, the data it provides is unnecessarily detailed for routine testing. It will be the lower-cost, more accessible diagnostic devices that will be used to test specific genetic sequences – leading to a proliferation of companion diagnostics.
We are just scratching the surface of personalised medicine, which is why Cambridge Design Partnership is working with both drug delivery and diagnostics clients to help them navigate this rapidly developing market. If you’d like to know more, get in touch via hello@cambridge-design.co.uk.

Dan Haworth
Partner