How to boil your egg perfectly every time – according to simulation
on LinkedIn:
Search ‘how to boil an egg’ on Google, and you get over three billion results, some telling you to put the egg in cold water after boiling to preserve the runny yolk. Intrigued, we decided to investigate the science behind this advice.
Rather than heading straight to our lab for experimentation, we used computer simulation to calculate and model the movement of heat and temperature through the egg and surrounding fluid. Simulation lets us predict data at times that would be impractical or expensive in actual experiments.
Modeling the heat flow in a boiling egg could be a surprisingly tricky problem. An egg consists of a solid shell holding the white and yolk, initially in a liquid state but solidifying as the cooking continues. Being natural products, the exact properties and sizes of eggs vary.
To simplify the problem, we found technical publications that describe the average dimensions and thermal properties of the shell, white, and yolk for a typical egg. We decided to define these properties at a temperature of 60°C, which is around the point the yolk starts to solidify. Using computer-aided-design software, we created the geometry of the egg, and defined a body of fluid to surround it. This fluid body represents the boiling water in a saucepan during the first cooking stage. Afterward, the fluid body can be used to mimic cool-down in air or a bowl of 10°C cold water. We decided that the eggs would start the process from room temperature in all cases.
We ran the simulation using powerful software, Ansys Fluent. The software was initially developed for understanding problems such as the flow of air over planes or heat in a chemical plant, but it can be applied to domestic problems such as the humble boiled egg. To allow the simulation to run quickly on an ordinary computer, we took advantage of the fact an egg shape is a body-of-revolution and looks the same however it’s rotated around its axis. This lets us model it as an axisymmetric body that the computer considers two-dimensional. This reduces the number of calculations and gives us the answer quicker and more cheaply than simulating the real-life, three-dimensional shape.
As an example of the simulation results, Figure 1 shows the temperature distribution on a slice along the egg’s axis after cooking in boiling water for six minutes. The material towards the outside has heated up close to the temperature of the water. However, the central region corresponding to the yolk is still around 50°C, corresponding to a runny egg.
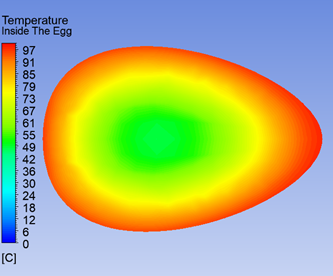
Figure 2 shows a side-by-side comparison of subsequently cooling the egg in air or 10°C water for five minutes (five minutes being our estimate of the time it takes to finish eating our first dippy egg and move on to the second). When cooled in air, the central region of the egg continues to increase to 70°C, removing the prospect of a runny egg, even though the outer region and shell have decreased in temperature. In contrast, after cooling in water, the central region stays unchanged at 50°C while the shell has decreased close to 10°C. Leaving your perfect dippy egg in air risks ruining the runny yolk – but cooling it in water may save it.
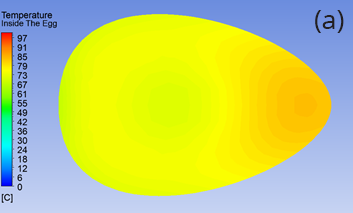
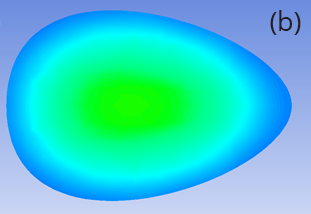
Figure 2: Temperature distribution on a slice through the egg following cooking and five minutes of cooling in (a) air and (b) water.
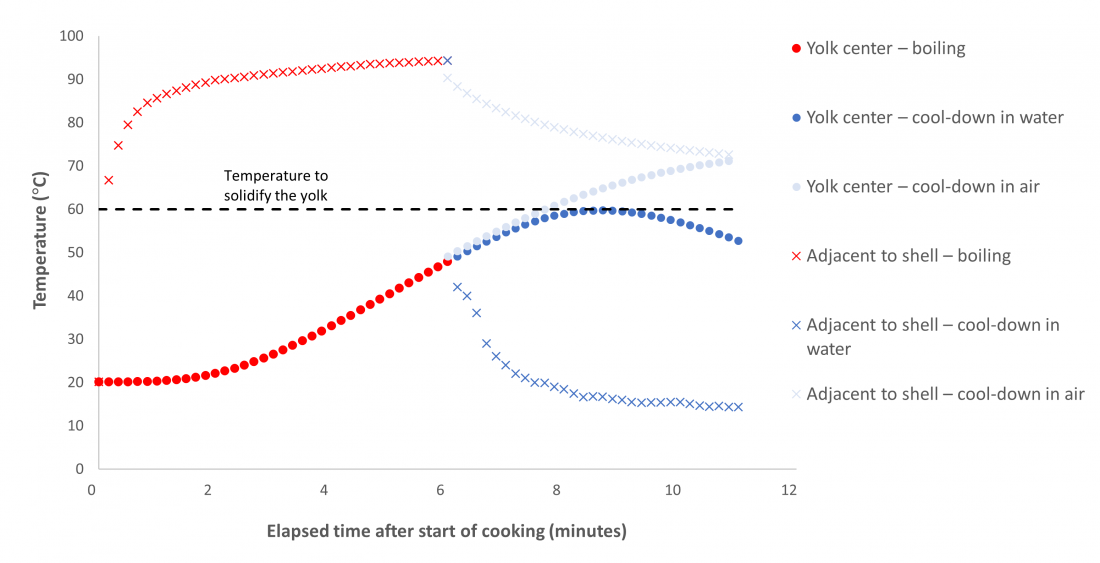
As well as modeling the overall temperature in the egg, we extracted the data for two specific points – at the center and the edge of the egg – and plotted them on a graph (Figure 3) to see how they differed. The data showed that the yolk’s temperature lags that at the shell. This is because the thermal diffusivity of the white and yolk are relatively low. Thermal diffusivity is a measure of how quickly heat can move through a material. So, it takes a while for the yolk to heat up, but once it does, it keeps cooking, absorbing heat from the rest of the egg material. It’s slow to respond to changes in the surrounding water (or air). The temperature just inside the shell responds much more quickly to changes, though, since the path the heat needs to travel from the surrounding fluid is considerably shorter, and the thermal diffusivity of the shell markedly higher.
With the aid of some considered simplifications, we think this simulation analysis has proven the cookery expert right: cooling eggs down in cold water really does preserve the runny yolk. However, whenever you analyze a problem for the first time, it’s important to compare results against an experimental benchmark, so you can confirm the realism of the assumptions and simplifications in a computer simulation. We took three eggs and boiled each for six minutes in a lab beaker. One was opened straight away, and the other two after cooling in cold water or in air for five minutes. As predicted by our computer simulation, the yolks ranged from runny to fully cooked. And the best thing about this experiment? Everyone got an egg cooked precisely to their liking at the end.
Our engineers and designers are enthusiastic about using science to understand and improve the processes and products we use every day. Get in touch with emma.lindsey@cambridge-design.com to learn more about our work in kitchen technology or simulation.

Jonathan Wilkins
Senior Consultant Mechanical Engineer

Emma Lindsey
Associate Mechanical Engineer