Case Study | Pelikan Technologies
Low pain lancing system for diabetics
Diabetes is a chronic disease that causes high blood sugar and afflicts over 400m people worldwide. Measuring blood sugar is a part of a patient’s regular routine to control their condition.
Pelikan Technologies aimed to disrupt the diagnostic market with a painless blood lancing system that would significantly improve a patient’s experience. We were selected to work with Pelikan’s R&D team to commercialise their lancing technology and bring their first medical device to market within two years.
Our approach
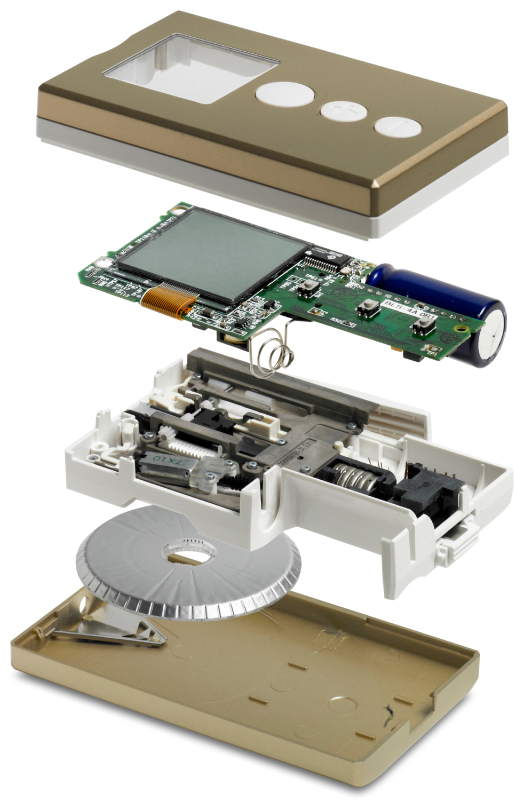
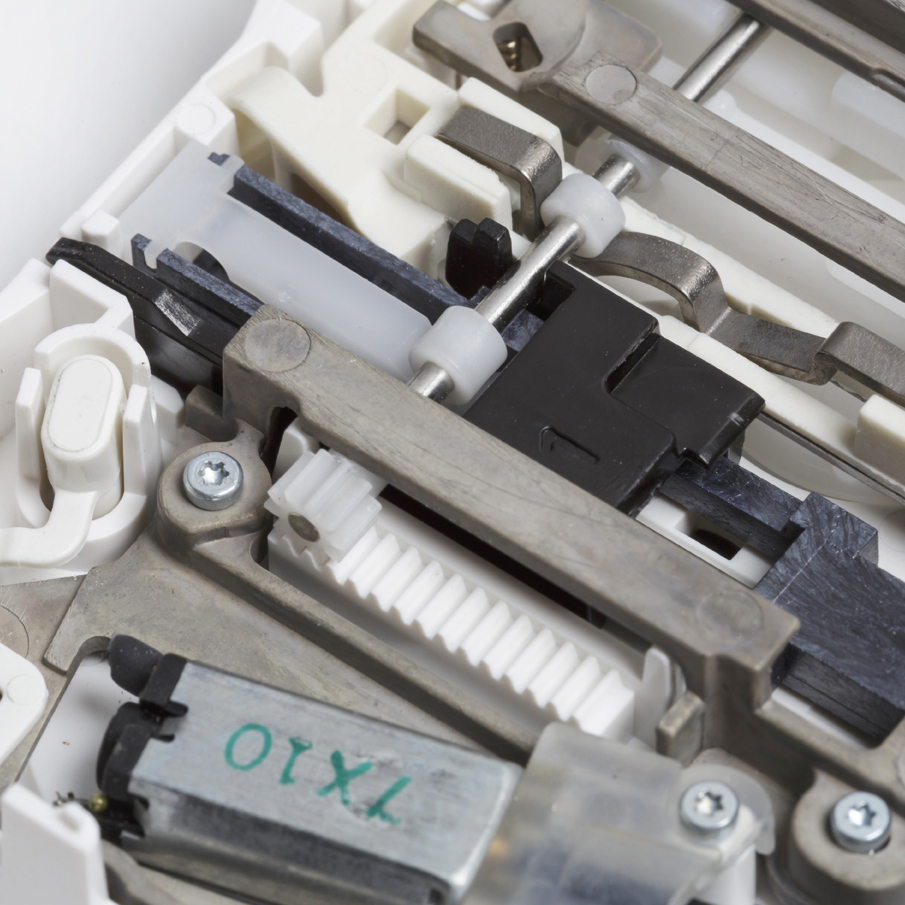
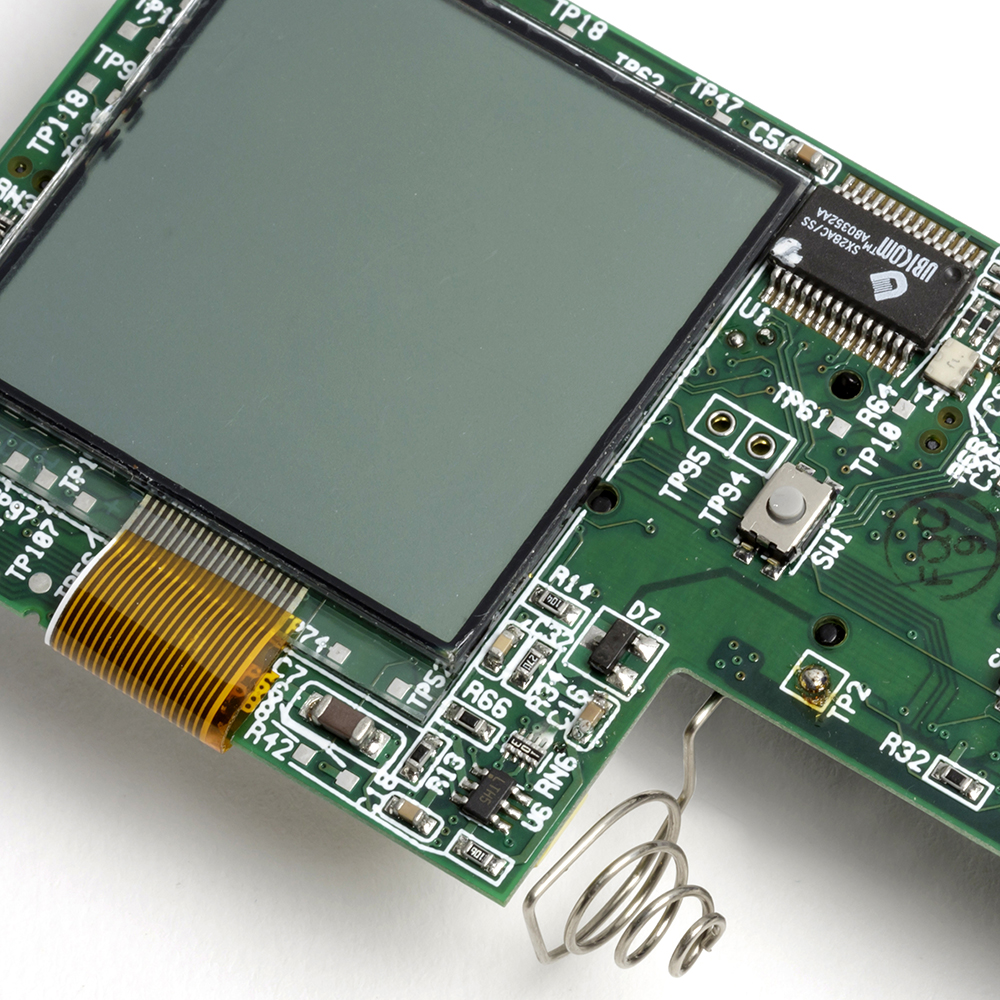
Implementing their technology in an approved medical device provided several challenges, the sector is highly patented and the low pain technology needed a very high performance drive system, well beyond what was commercially available. In addition, the lancet consumable had to be manufactured automatically in very high volume with an ultra low cost of goods.
In partnership with the Pelikan team we developed a low cost linear motor based lancet drive system with a high dynamic response and precision. We designed a disposable lancet cassette and manufacturing process that included a novel 3-dimensional foil sealing technology. Finally, we integrated these elements with a mechanical system to automatically load a fresh lancet into the electronic engine, and after use return it to avoid needle stick injury.
Design iterations from the first sketch model to finished production design.



The value we created
Implementing their technology in an approved medical device provided several challenges, the sector is highly patented and the low pain technology needed a very high performance drive system, well beyond what was commercially available. In addition, the lancet consumable had to be manufactured automatically in very high volume with an ultra low cost of goods.
In partnership with the Pelikan team we developed a low cost linear motor based lancet drive system with a high dynamic response and precision. We designed a disposable lancet cassette and manufacturing process that included a novel 3-dimensional foil sealing technology. Finally, we integrated these elements with a mechanical system to automatically load a fresh lancet into the electronic engine, and after use return it to avoid needle stick injury.
“Cambridge Design Partnership approached each of our projects with vision, insight and intelligent thinking. Their team really is dedicated to answering our brief, and they integrate well with our in-house engineers. CDP are super-knowledgeable in medical engineering.”