Case Study | QuantuMDx
From prototype to PCR testing system, fast
Innovation in a pandemic
Q-POC™ has the potential to improve millions of lives by detecting infectious diseases earlier, informing treatment decisions, and reducing rates of transmission.
QuantuMDx’s rapid sample-to-answer, multiplex polymerase chain reaction (PCR) and microarray testing system provides COVID-19 results in approximately 30 minutes from a nasal swab, at the point of need in settings such as hospitals, clinics, airports, care homes, events, and workplaces.
During the COVID-19 pandemic, Q-POC™ was needed urgently. Working closely with QuantuMDx, our multi-disciplinary team – over 30 mechanical and electronic engineers, prototyping, quality and clinical manufacturing experts in the UK and US – stepped up to accelerate the system’s development in just five months.
A challenge of utmost importance
As the world went into lockdown, QuantuMDx needed our help. The UK-based developer of point-of-need diagnostics had secured UK Government funding to develop Q-POC™ – transforming the prototype to an instrument designed to detect SARS-CoV-2 (the virus causing COVID-19 disease).QuantuMDx needed CDP’s support bringing Q-POC™ to market as quickly as possible. This required simplifying the system, improving reliability, and designing the system for manufacture – all in a regulated framework suitable for CE-IVD submission.

Speed through highly parallel working
Racing against the clock meant working linearly wasn’t an option; system and sub-system development activities had to happen in parallel, which meant risk mitigation was vital. Our experienced team assessed development effort and timescales against risk, triaging what we could improve. Some issues could be rectified with incremental improvements, others needed more radical changes. This demanded our team to quickly and methodically design, prototype and test – and commit to bold changes.

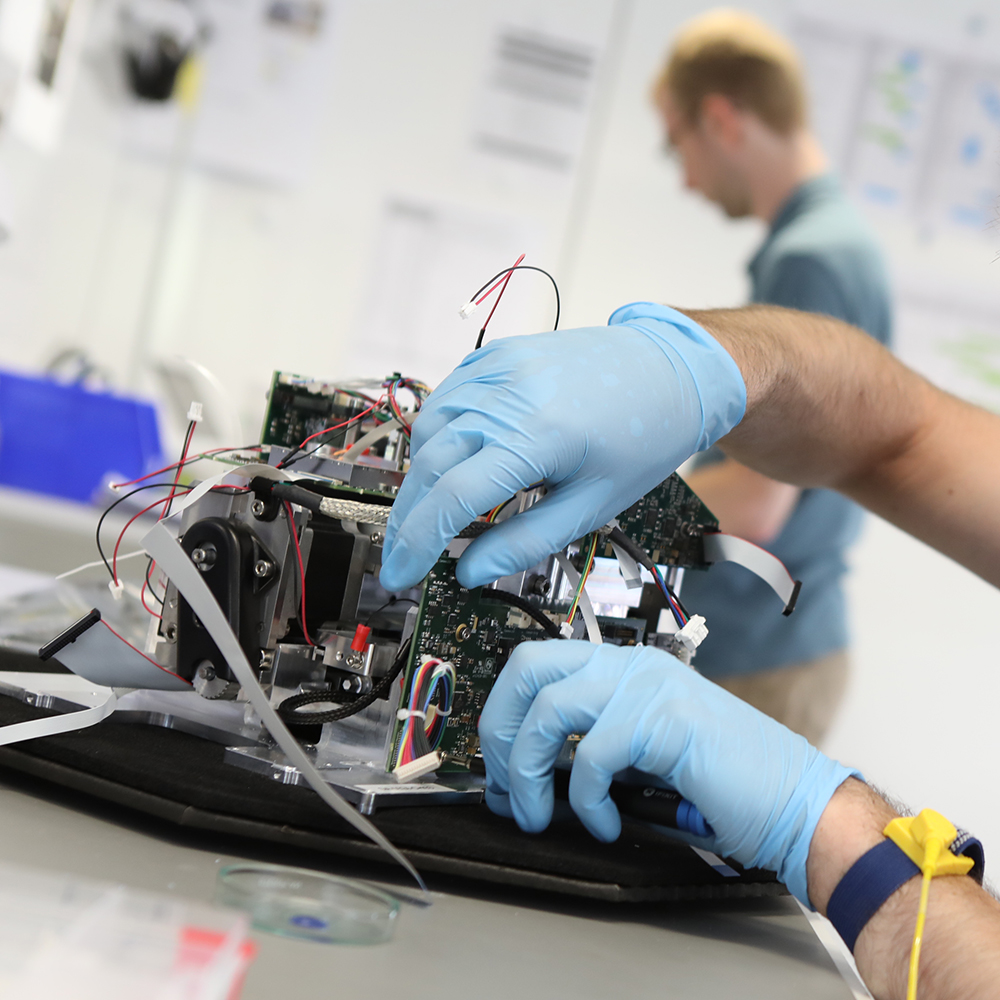
Seeing the future
Getting Q-POC™ to market under these circumstances meant stripping back some existing functionality that would not be needed for the SARS-CoV-2 assay, including several modules and components that would be later required for future assays. Our team worked closely with QuantuMDx, taking a long-term view so the instrument would still be suitable to upgrade in future for new assay functionality. This meant, designing a modular architecture, and ensuring the design intent would allow additional actuators, heaters, and cameras to be added later.

Regulatory expertise
Because Q-POC™ would become a regulated in vitro diagnostic medical device, QuantuMDx needed an innovation partner with a deep understanding of bringing medical devices to market. Our ISO 13485-certified Quality System includes short-run manufacturing which enabled us to manufacture pre-production devices for CE-IVD submission.
Complete chain of risk management was vital, including traceability of parts, detailed instructions for manufacture, and rigorous creation of quality documentation needed for regulatory submission. Our team managed working environments and controlled manufacturing space to ensure this project resulted in a stand-out example of an on-site clinical manufacturing line.
Beautiful engineering
Systems-level thinking meant the impact of small changes were considered across the whole system, resulting in beautiful engineering. We executed over 100 core design changes, including to the system’s chassis, which was initially constructed from parts that could flex and twist undesirably. We provided confidence in the device’s rigidity by switching to a one-piece, machined block, which could be later cast from aluminum. We improved the reliability of custom actuator assemblies as well as reducing the part count and size. We designed and manufactured new printed circuit boards (PCBs) rationalizing the number. We improved the cable management which led us to drastically simplify the assembly process and improve instrument reliability.
Across the finish line
Working at immense speed, we helped QuantuMDx across the finish line – manufacturing, testing, and delivering 45 complete instruments in just five months.
Putting our Potential Realized methodology into practice enabled us to deliver Q-POC™ as rapidly as possible, taking it from a prototype to a manufactured product.
In November 2021, QuantuMDx secured £15 million in equity funding to expand Q-POC™’s functionality and testing capabilities, including a multiplex respiratory panel and detection of sexually transmitted infections and human papillomavirus – building on the foundations of our work to create a true platform for future testing.


