News
CDP advances surgical robotic technology
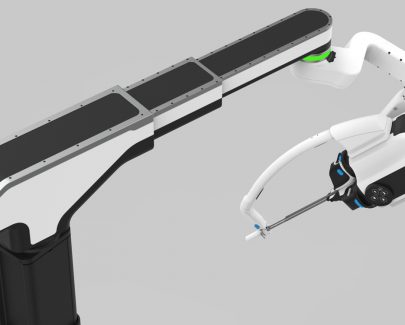
In collaboration with CDP, Titan Medical has unveiled its next-generation technology for single-access robotic-assisted surgery (RAS).

In collaboration with CDP, Titan Medical has unveiled its next-generation technology for single-access robotic-assisted surgery (RAS).

Having developed a highly effective liquid biopsy system to isolate CTCs, Vortex Biosciences needed our technical expertise and manufacturing experience to improve the system’s disposable cartridge for reliable performance and high-volume production.

Senior Consultant Jon Powell shares some of the obstacles encountered conducting pilot builds in-house to help our clients bring devices to market – and gives four pointers for ideal pilot manufacturing for clinical trials.

Getting medical devices regulated to international standards is not easy. Rose explains how this process could be made much simpler.

CDP’s partner QuantuMDx has now embarked on £11 million scale-up to mass manufacture of their Q-POC™ device.

WEBINAR How to unlock the potential of digital, to design products for the benefit of all stakeholders With Clare Beddoes 8 APR 2020 Done well, digital can enhance the development of healthcare devices and help provide benefit to all stakeholders. But before applying digital as a tool to your device…

WEBINAR Human factors in your hands: Usability for more than just regulatory compliance With Louise Place and Lucy Sheldon 25 FEB 2020 The world of medical devices has been combining user experience and the understanding of patient capability since the advent of IEC 62366, and the US Food and Drug…

Cambridge Design Partnership is recognised as one of the top three agencies in Europe and the Americas for design innovation, according to the Red Dot awards programme. Red Dot has become established internationally as one of the most sought-after seals of quality for good design. They organise annual competitions looking to applaud…

For the last decade I have been part of the medical devices industry, most recently as part of a design consultancy firm specialising in medical device innovation. In the last few years our world has been shaken with reports of the failure of medical device implants and the insinuation of…

The recent Digital Health World Congress 2018, which was held in London at the end of November, covered aspects of medical and mobile technology. It certainly presented an interesting and diverse line-up of speakers and exhibitors. A key learning we took from the event is that digital health (the intersection…
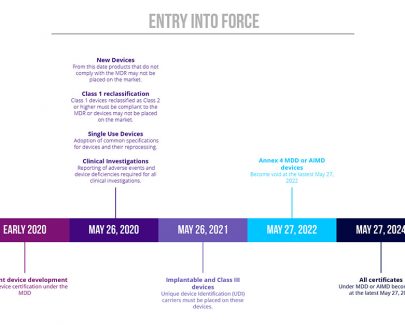
More than a year has passed since 25th May 2017 and the start of the three-year transition period from the Medical Devices Directive (93/42/EEC) and Directive 90/385/EEC on active implantable medical devices (AIMDD) to the Medical Devices Regulation (EU 2017/745).But what does this mean for businesses with products currently on…

24 September 2018 – A doctor’s experience of dealing with acute trauma on the battlefield is being used to help improve the lives of critically ill civilian patients in intensive care units (ICUs). Dr Charlotte Small and the critical care research team at the Queen Elizabeth Hospital Birmingham (QEHB) in the…