Case Study | Quantii
Multi-application sensor platform to analyze patient behaviour

Billions of dollars are invested each year in clinical studies for new respiratory therapies. If the data collected turns out to be of poor quality or inconclusive then the study can fail. The unfortunate result is that potentially life saving therapies may never make it into the hands of patients.
Inhalers, for example are notoriously tricky to use and their performance is typically dependent on how the patient inhales. Even in monitored studies only a qualitative assessment of technique is possible. In Phase III studies, which are often unsupervised, the only opportunity to explain any unexpected or outlying data has been to rely on Case Report Forms.
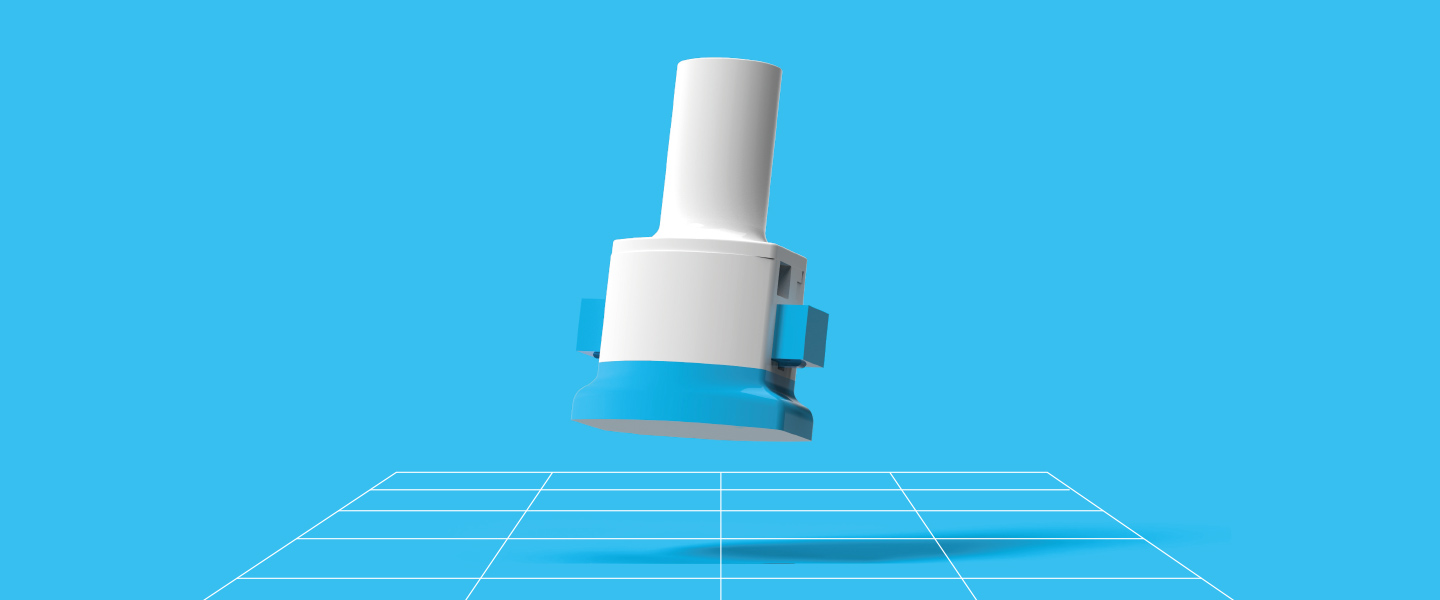
Our approach
We created the quantii sensor platform to improve data quality in product trials, by improving training and providing rich data on patient interactions.
We incorporated discreet, miniature sensor technology into the test device together with data storage and wireless communications. Firstly, user training is enhanced as quantii provides real-time feedback so users can quickly understand what is needed of them and practice the correct technique. During the trial each operation of the device is monitored and translated into a wealth of quantified user insights, such as air flow and capsule discharge rates throughout each inhalation cycle.

Quantii provides a wealth of insight, including accurate measurement of the inspiratory flowrate profile, ramp-up rate, total inhaled volume and the spin rate of the capsule
The value we created
Quantii enables high-quality, data-driven clinical studies and, for the first time, use error can be distinguished from drug efficacy. This allows you to direct remediation investment to the right places and accelerate your journey to market.