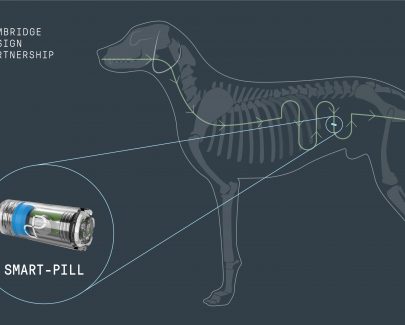
Analysis
CDP engineer creates a remarkable musical instrument
Jonathan Morris, Mechanical engineer, explains how he came to design and build a most unusual musical instrument at CDP Q. CDP doesn’t usually make musical instruments, does it?Jonathan: No! This is definitely one of our more unexpected projects here at Cambridge Design Partnership. But when composer Robert Laidlow approached us…