
Analysis
AI in healthcare, separating facts from fiction
James Baker, partner at Cambridge Design Partnership, considers the future for AI in the real world with help from a sideways look at its portrayal on the big screen.

James Baker, partner at Cambridge Design Partnership, considers the future for AI in the real world with help from a sideways look at its portrayal on the big screen.

Karla shows us how modelling renal vasculature can help better understand CKD.
It may seem like there is plenty of time before the new IVD (in vitro diagnostics) regulation (IVDR) [EU 2017/746] comes into effect. The deadline for transfer is 2 years later than that for medical devices [EU 2017/745], so May 2022 might seem distant. However, given the changes that are…

RAPS (Regulatory Affairs Professionals Society) publish a set of excellent “Fundamentals” books, each covering a different regulatory context: US, EU, Canadian and International (which covers other markets), (see here). These books detail the key aspects of the regulations for pharmaceuticals, medical devices and IVDs (In vitro diagnostics) with considerations about…
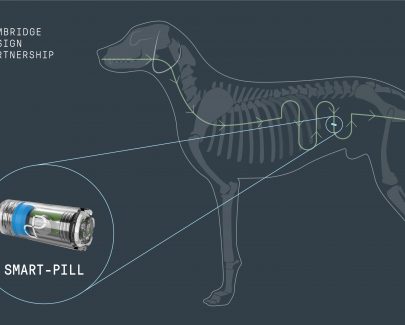
A team from Cambridge Design Partnership has created a ground-breaking ‘smart pill’ to gather crucial nutritional information to help develop innovative new pet foods. CDP scientists and engineers worked with the world-renowned Waltham Centre for Pet Nutrition on an electronic pill to collect food samples inside the canine gut during…

In our recent blog post ‘What’s Next In Hemodialysis’ we looked at some of the trends that are driving the industry. This month we report from the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) congress in Budapest. With more than 110 companies exhibiting to 9,500 attendees, Matt…

The 56th European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) conference takes place this year on June 13-16 in Budapest. With this in mind, Matt Brady, Partner & Head of Medical Therapy at Cambridge Design Partnership and Jess Carroll, Mechanical Engineer from Cambridge Design Partnership consider the future of renal disease patient…

Matt & Jess consider the future of renal disease patient care, and how the growing home dialysis market could drive innovation.

Biosimilars are taking on the multi-billion-dollar blockbuster drugs of today, with some sources suggesting that the market will be worth greater than $20 billion per annum within the next couple of years. The growth is being driven by factors such as healthcare systems looking to reduce costs and the growing…

For the last decade I have been part of the medical devices industry, most recently as part of a design consultancy firm specialising in medical device innovation. In the last few years our world has been shaken with reports of the failure of medical device implants and the insinuation of…

This article was first published on Drug Development and Delivery.“Those who expect moments of change to be comfortable and free of conflict have not learned their history.” For many involved in the medical and pharmaceutical industries within the last few years, this quote – attributed to American historian Joan Wallach…
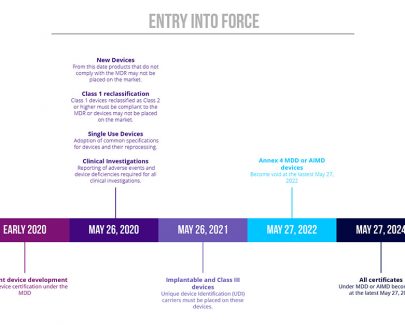
More than a year has passed since 25th May 2017 and the start of the three-year transition period from the Medical Devices Directive (93/42/EEC) and Directive 90/385/EEC on active implantable medical devices (AIMDD) to the Medical Devices Regulation (EU 2017/745).But what does this mean for businesses with products currently on…