
Whitepaper
A Strategic Technology Roadmap for the UK In Vitro Diagnostics Industry
Our Roadmap defines the key technologies and strategies that can place the UK at the forefront of the IVD industry.

Our Roadmap defines the key technologies and strategies that can place the UK at the forefront of the IVD industry.

We designed and tested over 100 improvements to our client’s instrument in just five months, enabling rapid iteration and scaled-up production. In addition, our team manufactured 45 units and generated the device history records needed to gain a CE Mark.

Having developed a highly effective liquid biopsy system to isolate CTCs, Vortex Biosciences needed our technical expertise and manufacturing experience to improve the system’s disposable cartridge for reliable performance and high-volume production.

Join Martha Hodgson and Jessica Platt and explore how the power of inclusive, experience-centered design and smart application of technology has accelerated the rise of FemTech.
Partnership aims to ensure that transformative diagnostic and healthcare technologies reach patients fast.
James explores how blockchain is moving away from its cryptocurrency roots, providing a tool for solving challenges in numerous real-world applications.
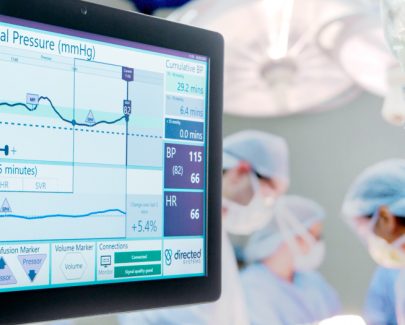
CDP play a key role in pioneering clinical support software solution to assist clinicians during surgery.
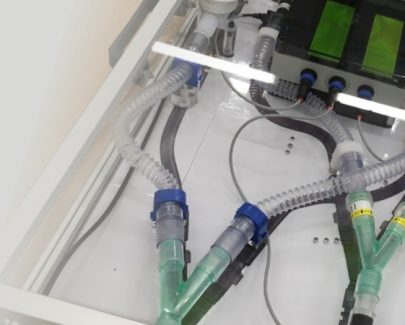
Staff at CDP assist innovative ventilator-splitting project in response to the Covid-19 emergency

WEBINAR Improving drug delivery systems for self-administration With Bastiaan deLeeuw, Uri Baruch, Clare Beddoes and Chris Houghton 8 JUN 2020 As self-administration in the home setting grows in importance, our panel of experts discuss the continuous drive to design and improve drug delivery systems for self-administration and home-use. Enjoy the full…
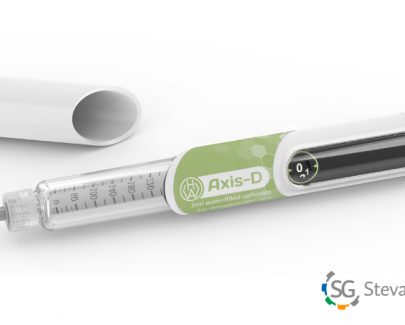
The collaboration strongly supports the expansion of the Stevanato group’s portfolio of devices for patients suffering from diabetes.

WEBINAR How to unlock the potential of digital, to design products for the benefit of all stakeholders With Clare Beddoes 8 APR 2020 Done well, digital can enhance the development of healthcare devices and help provide benefit to all stakeholders. But before applying digital as a tool to your device…

WEBINAR Human factors in your hands: Usability for more than just regulatory compliance With Louise Place and Lucy Sheldon 25 FEB 2020 The world of medical devices has been combining user experience and the understanding of patient capability since the advent of IEC 62366, and the US Food and Drug…