Case Study
A novel wearable that saves lives
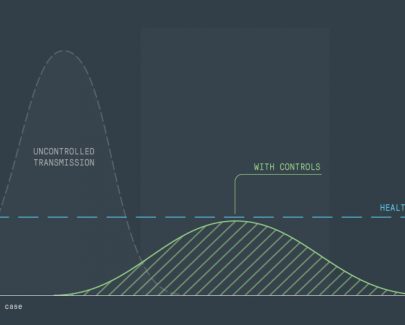
In an intense disaster or battlefield scenario, correctly identifying which victim to treat first can be a matter of life or death, so we created a specialised wearable monitor. The device won the prestigious ‘Best of the Best’ Red Dot award.